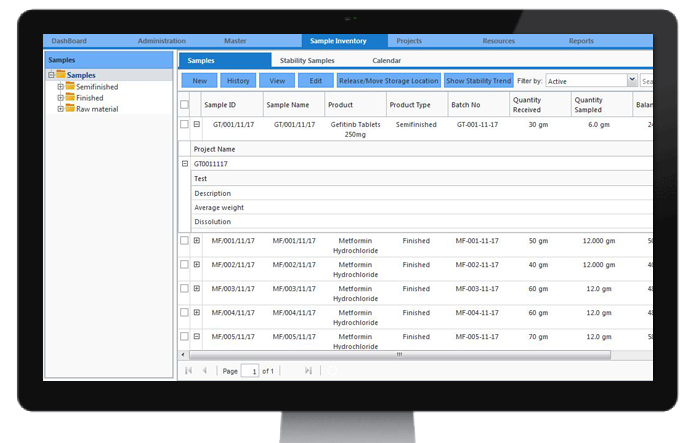
Sample Management
The location of samples and their movement within and between laboratories can be recorded, providing a full chain of custody. This is especially important when handling very expensive or sensitive materials. Storage Location Management can provide a visual mechanism for seeing where samples are being stored, where they have been and other pertinent data. Knowing where samples are makes life easier, particularly when finding specific samples for analysis for processing.
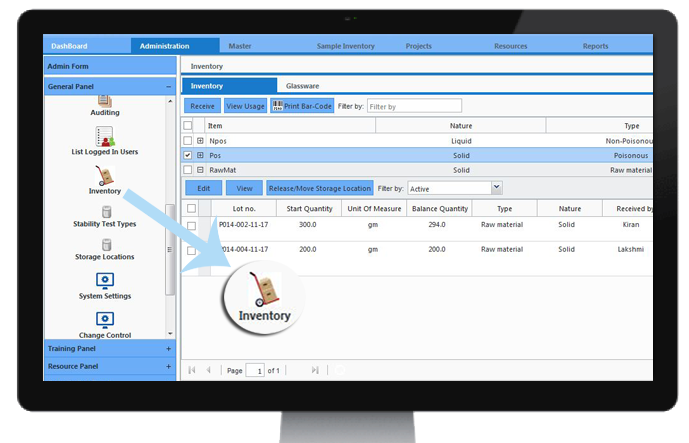
Inventory Management
LIMS enables laboratory inventory to be managed - for example, products for shelf-life testing, glassware, spares, and consumables. Every time a new batch of standard/reagent/media is prepared it can be added automatically to the inventory. Minimum stock levels can be monitored to ensure testing resources are available when required.
Workflow Management
This involves the definition of the flow of samples through the laboratory and the procedures to be performed. CodonLIMS can provide the company with the ability to approve important LIMS records using a flexible review and approval mechanism. This includes routing and recording the approval to multiple individuals within the organization.
Integrating with ERP and External Systems
Final reports generated by the laboratory as an end-product is a certificate of analysis (COA), but LIMS may be required to provide information to external systems or other departments within the company or for external laboratories. In the manufacturing environment, it is common for results to be sent securely to production departments for use in their process control systems.
Instrument Integration
Integrating instruments can reduce typographical and transcription errors. By interfacing instruments directly to LIMS, data is tracked electronically.
CodonLIMS is equipped with LIMSinc - An Codon instrument integration tool which acts as a bridge between LIMS and instruments. LIMSinc reads electronically generated instrumentation data, filters error data and sends valid data to LIMS for processing, analysis and storage for further reference.
All data flow from instruments to LIMSinc and LIMSinc to CodonLIMS is tracked and audited on respective audit modules.
Stability Studies
Shelf-life studies, which are common in pharmaceutical and food industries, provide information on how stored products will behave at defined time periods to ensure safe usage. Protocols can be configured to meet many varied demands for shelf-life testing with tests being applied at various time-points and conditions. Stability studies can be applied to different types of storage samples along with storage conditions.
Barcode (and RFID)
Organisations need to track and manage critical factors like inventory and samples from warehouse to laboratory till the finished product transported out of the production facility. CodonLIMS handles this with a customizable built-in integrated barcode generator module, which generates and prints barcodes separately for every aspect based on user requirement.Due to which, the time consumption for tracking and maintaining the records will be minimized, resulting in reduced labor costs, increase in labor and production process efficiency.
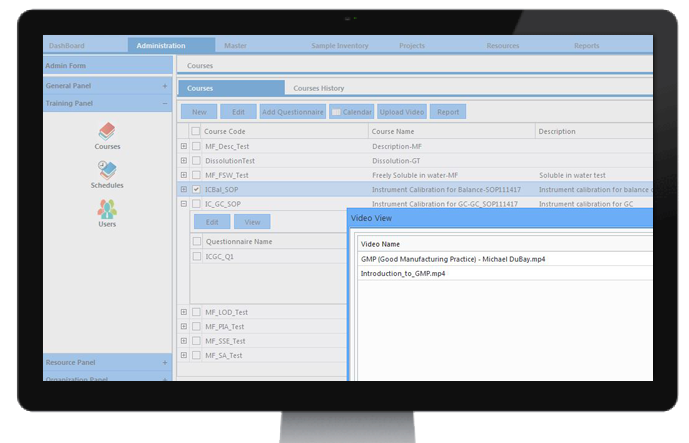
Training Management
The pharmaceutical domain is governed by a variety of guidelines and regulations to ensure the safety and effectiveness of its products. In addition to SOP (Standard Operating Procedure) training and cGMP (Current Good Manufacturing Practices) training, Safety and Environment training is also mandatory to ensure that employees are equipped to work within the industry guidelines. Since the training process is continuous, it becomes necessary to record and document all conducted training for the purpose of detailed audits.
Benefits of Training Management Systems
Apart from maintaining compliance, a Training Management System based solution can be extremely efficient to when managing industry mandatory training requirements.
Alerts can be automatically sent through emails or SMS as soon as a trainee has been assigned to a scheduled course. The frequency of these notifications can be customized as per need. CodonLIMS can be customized to conduct the analysis on a continuous basis or from time to time.
Instant approval tools can facilitate the creation of e-courses on short notice, allowing the repository of available documents to grow rapidly. Updates and changes can also be made on these courses very easily, using the same tools.
Reports of training planned and completed for each employee are available at all times, easing the audit process. Online assessments for courses can be conducted with the help of MCQs (Multiple Choice Questions). Records of these evaluations are available at all times for audit purposes.
CodonLIMS Training Management System not only helps organizations comply with mandatory training but also helps to increase efficiency in achieving excellence in product quality.
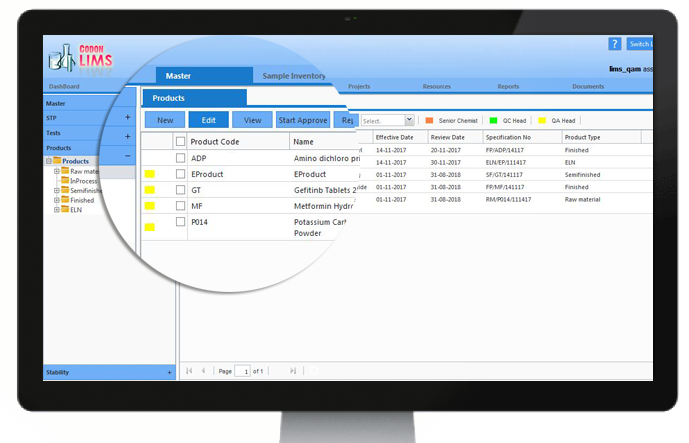
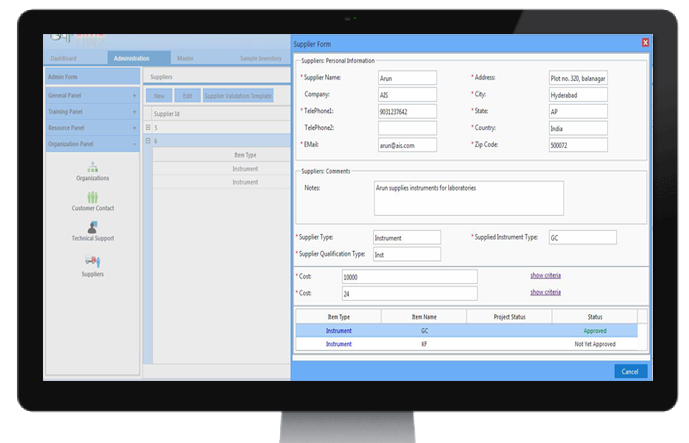
Supplier Validation:
Supplier validation/qualification gives the peace of mind to the organization as well as to the customers This reduces the risk factors of problems emerging while in production before the actual product is procured. This results in minimizing loss of physical and financial resources.
CodonLIMS comes with a customizable Supplier Validation module, by which user can compare qualification results and select the best suitable supplier for their needs.
CodonLIMS Supplier Validation module can validate and store information about suppliers including raw materials, inventory and instruments separately, and can be extended to other factors like SOPs etc.
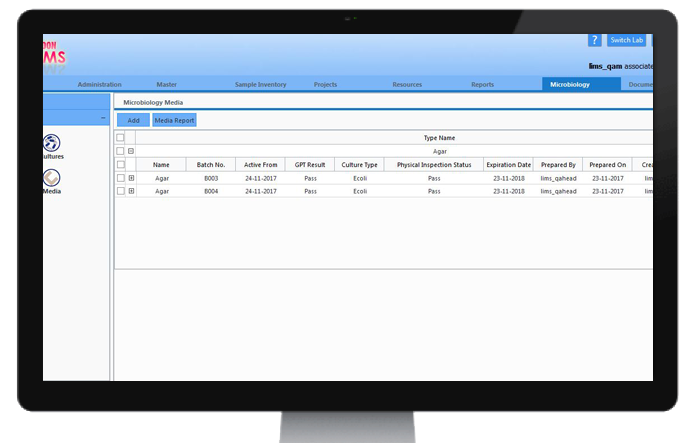
MicrobiologyLab Management
Microbiology lab activities contribute significantly in the production and quality control of antibiotics in the pharmaceutical industry. CodonLIMS has an excellent microbiology module to maintain records of culture and media preparation and their usage tracking.
Using this module, microbial tests for growth promotion, sterility, water testing, microbial count etc, can be designed, analyzed and reports can be generated for the results observed.
These results can be approved and stored instantly by the authorized personnel of the labs for further processing.
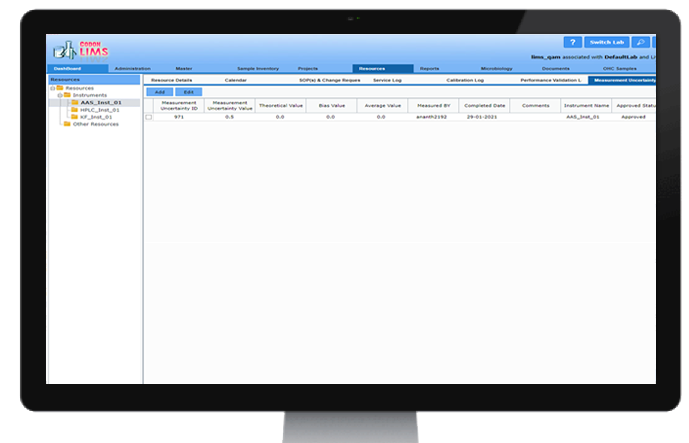
Measurement of Uncertainty
CodonLIMS provides platform to capture the measurement of uncertainty data for each instrument / test equipment along with final uncertainty range. This data is made available when using instrument for any measurement and the results can be shown along with the uncertainty range.
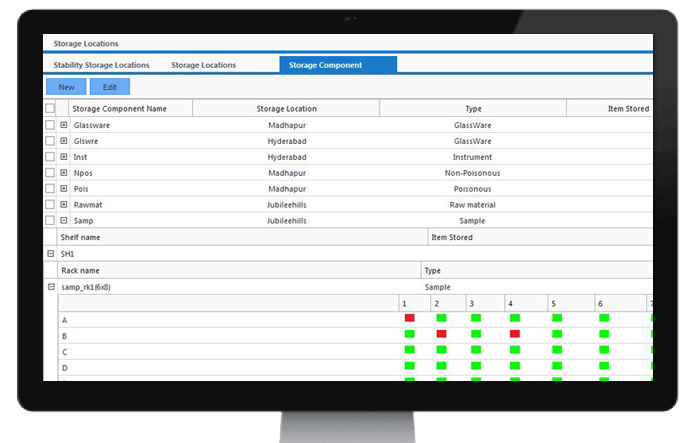
Storage Locations
Organisations need to track items like Inventory Consumables, Instruments, Samples etc in their facility. It takes a lot of effort in finding the available places for storing these items in the inventory for the Organisations having a humongous number of storage locations. CodonLIMS makes these tasks easier by providing a rich graphical interface which provides a convenient way for the user to visualize storage locations and to quickly identify available storage space so that user can store the items in appropriate places with ease. CodonLIMS supports storing the items in Multiple level storage hierarchy like locations, components, shelves, and racks etc. Using the Application we can keep track of items arrival to the inventory, placement of items to available locations, movement of items from one location to other location and removing the items from the location when items are expired(No longer needed). The application also stores location's attributes such as temperature, humidity etc. So that user can check whether the items that are going to be stored are suitable for the respective location.
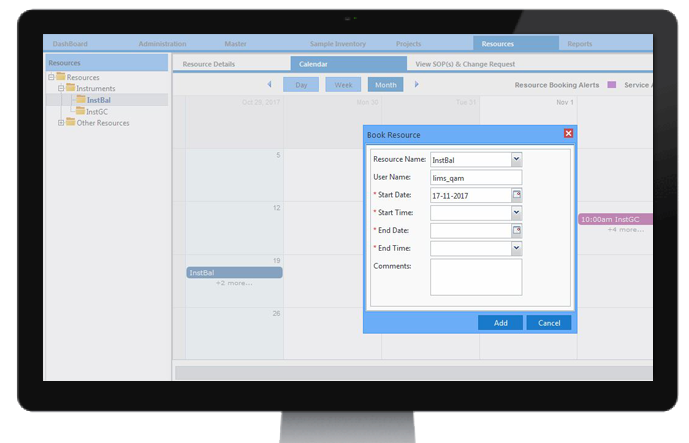
Resource Booking
In any Industry, controlling and optimizing work and workloads on resources like laboratory equipment, conference/discussion rooms, projectors etc. plays a vital role in improving the effective utilization of resources.
CodonLIMS provides a built-in role-based resource scheduling and booking system that enables the user to effectively manage the reservations in a calendar view. Flags and notifications for samples allotment to an instrument, instrument pre-booking, instrument service and calibration schedules and reservations for any conference room are available to the organization.
Through the CodonLIMS instrument schedules calendar view, a user can have an overall pictorial representation of workload and efficiency of each instrument. Online resource booking provides a quick response to schedule requests for rooms and reduces the time taken by room moderators for managing room reservation requests.
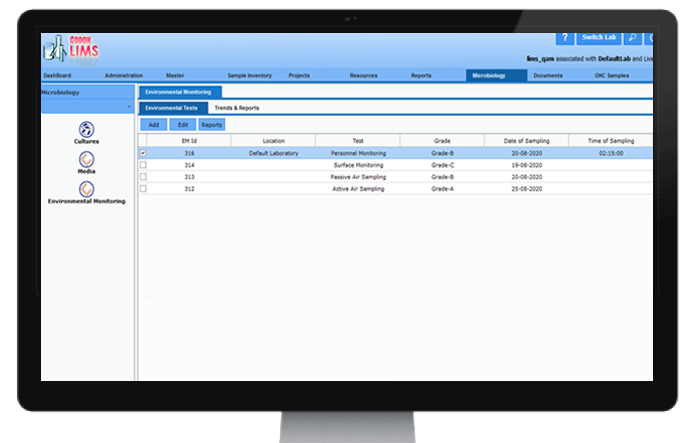
Environmental Monitoring
CodonLIMS provides platform to capture the data of Environmental Monitoring parameters across multiple locations of a plant. By using the data collected based on predefined tests, CodonLIMS can generate reports to help understand on-ground situation and take precautions accordingly for smooth functioning of production facilities.
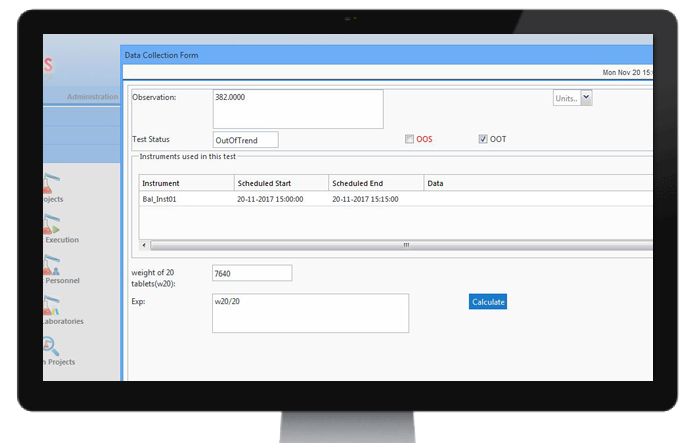
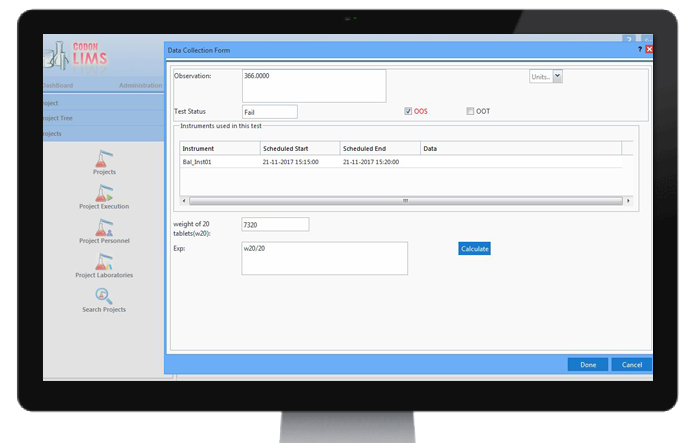
OOS study
CodonLIMS can automatically notify the user for Out of Specification (OOS) results if the test result
falls outside the specifications or acceptance criteria specified in the official Specification Documentation
Monographs or the finished product specification in registration pharmacopoeia.
Based on each individual organization standard operating procedures, for the workflow of OOS study, there is a feasibility for users to choose between resampling, re-analysis or re-assignment in multiple stages for the OOS investigation.
OOTStudy
CodonLIMS calculates the out-of-trend (OOT) result that does not follow the expected trend using 3-sigma model or regression control. Comparison are made with previous results collected from past and can be used to generate trends.